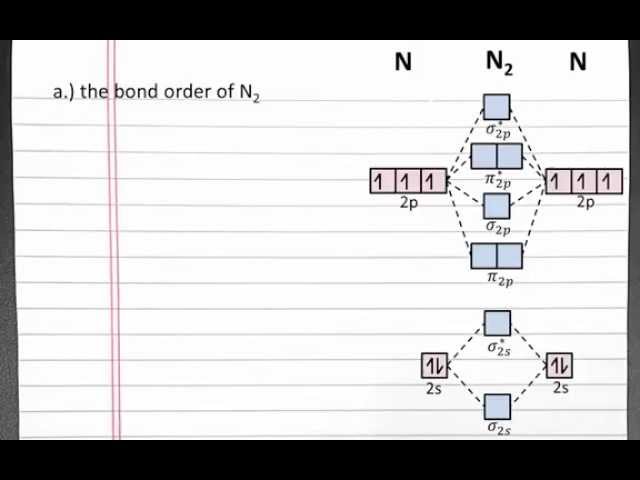
Determining the bond order of nitrogen molecules (N2) is vital in understanding the structure and reactivity of this essential element. Bond order measures the strength and multiplicity of chemical bonds between atoms, and in the case of N2, it plays a crucial role in its stability and inertness.
Comprehending the bond order of N2 has numerous applications in fields such as chemistry, materials science, and biochemistry. It enables researchers to predict molecular properties, design new materials, and understand the mechanisms of reactions involving nitrogen. One notable historical development was the discovery of the triple bond between nitrogen atoms in N2, which revolutionized our understanding of chemical bonding and molecular structure.
In this article, we will delve into the detailed steps of calculating the bond order of N2, exploring its significance, and examining its implications in various scientific disciplines.
How to Calculate Bond Order of N2
Determining the bond order of nitrogen molecules (N2) is crucial for understanding their stability, reactivity, and numerous applications. Key aspects to consider when calculating the bond order of N2 include:
- Molecular orbital theory
- Valence electrons
- Bond length
- Bond strength
- Resonance
- Hybridization
- Magnetic properties
- Spectroscopic data
Understanding these aspects enables chemists to accurately calculate the bond order of N2, which provides valuable insights into its chemical behavior. For example, the triple bond between nitrogen atoms in N2, indicated by a bond order of 3, contributes to its exceptional stability and low reactivity. This knowledge is essential for designing nitrogen-based materials, fertilizers, and pharmaceuticals.
Molecular Orbital Theory
Understanding molecular orbital theory is fundamental when calculating the bond order of N2. Molecular orbital theory describes the behavior of electrons in molecules by considering them as occupying specific orbitals that are formed by the combination of atomic orbitals. These molecular orbitals have different shapes and energies, and the electrons occupy them in a specific order based on their energy levels.
- Atomic Orbitals: Atomic orbitals are the orbitals of individual atoms, and they combine to form molecular orbitals.
- Linear Combination of Atomic Orbitals (LCAO): Molecular orbitals are formed by the linear combination of atomic orbitals.
- Bonding and Antibonding Orbitals: Bonding orbitals result from the constructive overlap of atomic orbitals, leading to electron density between the nuclei, while antibonding orbitals result from their destructive overlap, leading to nodal planes between the nuclei.
- Molecular Orbital Energy Levels: The energy levels of molecular orbitals are determined by the energies of the atomic orbitals that combine to form them.
Molecular orbital theory provides a framework for understanding the electronic structure of molecules, which is essential for calculating bond order. By considering the number of bonding and antibonding electrons, we can determine the bond order and predict the stability and properties of the molecule.
Valence Electrons
Valence electrons play a critical role in determining the bond order of N2. Valence electrons are the electrons in the outermost shell of an atom, and they are responsible for chemical bonding. In the case of N2, each nitrogen atom has five valence electrons. These valence electrons interact to form molecular orbitals, which are the orbitals that describe the behavior of electrons in the molecule.
The number of bonding and antibonding electrons in the molecular orbitals determines the bond order. Bonding electrons are electrons that occupy bonding orbitals, which are orbitals that result from the constructive overlap of atomic orbitals. Antibonding electrons are electrons that occupy antibonding orbitals, which are orbitals that result from the destructive overlap of atomic orbitals. The bond order is calculated as half the difference between the number of bonding and antibonding electrons.
In the case of N2, there are six bonding electrons and two antibonding electrons. Therefore, the bond order of N2 is 2. This indicates that there is a triple bond between the two nitrogen atoms in N2, which is consistent with the observed stability and low reactivity of this molecule.
Understanding the relationship between valence electrons and bond order is essential for predicting the properties and reactivity of molecules. By considering the number of valence electrons and the molecular orbital interactions, chemists can gain valuable insights into the behavior of molecules and design new materials with desired properties.
Bond length
Bond length is a critical component of how to calculate the bond order of N2. Bond length refers to the distance between the nuclei of two bonded atoms. It is an important parameter that provides insights into the strength and nature of the chemical bond. In the case of N2, the bond length is a crucial factor in determining the bond order.
The bond length of N2 is 109 pm, which is relatively short compared to other single bonds between nitrogen atoms. This short bond length indicates a strong bond between the two nitrogen atoms. The bond order of N2 is 3, which corresponds to a triple bond. The triple bond is characterized by a short bond length and high bond strength.
Understanding the relationship between bond length and bond order is essential for predicting the properties and reactivity of molecules. By considering the bond length and bond order of N2, chemists can gain valuable insights into the stability and inertness of this molecule. This knowledge is important for designing new materials, fertilizers, and pharmaceuticals that utilize the unique properties of nitrogen.
Bond strength
Bond strength is a fundamental aspect of how to calculate the bond order of N2. It refers to the strength of the chemical bond between the two nitrogen atoms. The bond strength of N2 is a key factor in determining its stability and reactivity, and it can be quantified using various methods.
- Bond dissociation energy: The bond dissociation energy is the energy required to break a bond between two atoms. It is a measure of the bond strength, and it is typically reported in units of kilojoules per mole (kJ/mol).
- Bond length: The bond length is the distance between the nuclei of two bonded atoms. It is an indirect measure of bond strength, with shorter bond lengths indicating stronger bonds.
- Force constant: The force constant is a measure of the stiffness of a bond. It is related to the frequency of vibrational motion of the bond, and it can be used to calculate the bond strength.
- Quantum mechanical calculations: Quantum mechanical calculations can be used to calculate the bond strength of N2. These calculations take into account the electronic structure of the molecule, and they can provide accurate estimates of the bond strength.
Understanding the bond strength of N2 is essential for predicting its properties and reactivity. By considering the bond strength, chemists can gain valuable insights into the stability and inertness of this molecule. This knowledge is important for designing new materials, fertilizers, and pharmaceuticals that utilize the unique properties of nitrogen.
Resonance
Resonance is a fundamental concept in chemistry that describes the delocalization of electrons within a molecule. It plays a crucial role in determining the bond order of N2 and is essential for understanding its stability and reactivity. Resonance structures are hypothetical Lewis structures that represent the different possible electron arrangements within a molecule, and they contribute to the overall bonding scheme.
In the case of N2, resonance structures can be drawn to show the delocalization of the electrons in the triple bond. These resonance structures contribute to the overall stability of the molecule by lowering its energy. The resonance hybrid, which is a weighted average of the resonance structures, is a more accurate representation of the electronic structure of N2 than any single resonance structure.
Understanding resonance is critical for accurately calculating the bond order of N2. The bond order is a measure of the strength and multiplicity of the chemical bond between the two nitrogen atoms. It is calculated based on the number of bonding and antibonding electrons in the molecular orbitals. Resonance structures help to identify the bonding and antibonding electrons, which are essential for determining the bond order.
In summary, resonance is a key concept in understanding how to calculate the bond order of N2. It provides a more accurate representation of the electronic structure of the molecule and helps to explain its stability and reactivity. By considering resonance, chemists can gain valuable insights into the behavior of N2 and its applications in various scientific disciplines.
Hybridization
In chemistry, hybridization refers to the mixing of atomic orbitals to form new hybrid orbitals with different shapes and energies. It plays a crucial role in determining the molecular geometry and bonding properties of molecules, including N2.
In the case of N2, the nitrogen atoms undergo sp hybridization. This means that one 2s orbital and three 2p orbitals from each nitrogen atom hybridize to form four equivalent sp hybrid orbitals. These sp hybrid orbitals then overlap to form a sigma bond ( bond) between the two nitrogen atoms.
Understanding hybridization is critical for accurately calculating the bond order of N2. The bond order is a measure of the strength and multiplicity of the chemical bond between the two nitrogen atoms. It is calculated based on the number of bonding and antibonding electrons in the molecular orbitals. Hybridization helps to identify the bonding and antibonding orbitals, which are essential for determining the bond order.
In summary, hybridization is a fundamental concept in understanding how to calculate the bond order of N2. It provides insights into the electronic structure of the molecule and helps to explain its stability and reactivity. By considering hybridization, chemists can gain valuable insights into the behavior of N2 and its applications in various scientific disciplines.
Magnetic Properties
Magnetic properties are essential considerations when studying the bond order of N2. Understanding the magnetic behavior of N2 provides valuable insights into its electronic structure and bonding characteristics.
- Susceptibility: The magnetic susceptibility of N2 is diamagnetic, indicating that the molecule is repelled by magnetic fields. This observation is consistent with the absence of unpaired electrons in the N2 molecule, which would otherwise give rise to paramagnetic behavior.
- NMR Spectroscopy: Nuclear magnetic resonance (NMR) spectroscopy can be used to probe the electronic environment of nitrogen atoms in N2. The 14N nucleus has a nuclear spin of 1, and its NMR spectrum provides information about the bonding and hybridization of the nitrogen atoms.
- Electron Paramagnetic Resonance (EPR): EPR spectroscopy is a powerful tool for studying paramagnetic species. However, since N2 is diamagnetic, EPR spectroscopy is not directly applicable to N2.
- Magnetic Moments: The magnetic moment of a molecule is a measure of its magnetic strength. N2 has a zero magnetic moment, which is consistent with its diamagnetic nature and the absence of unpaired electrons.
In summary, the magnetic properties of N2 provide valuable information about its electronic structure and bonding characteristics. The diamagnetic nature of N2, its NMR spectrum, and its zero magnetic moment are all consistent with the triple bond between the two nitrogen atoms. Understanding these magnetic properties is essential for accurately calculating the bond order of N2 and for comprehending its chemical behavior.
Spectroscopic data
Spectroscopic data play a crucial role in determining the bond order of N2. Spectroscopic techniques, such as ultraviolet-visible (UV-Vis) spectroscopy, infrared (IR) spectroscopy, and nuclear magnetic resonance (NMR) spectroscopy, provide valuable information about the electronic structure, vibrational modes, and nuclear spin properties of molecules. This information can be used to infer the bond order of N2 and understand its chemical bonding characteristics.
For example, UV-Vis spectroscopy can be used to measure the electronic transitions of N2. The energy difference between the ground state and excited states corresponds to the bond strength and can be used to calculate the bond order. IR spectroscopy, on the other hand, can provide information about the vibrational frequencies of N2. The vibrational frequencies are related to the bond strength and can also be used to calculate the bond order. NMR spectroscopy can provide insights into the nuclear spin properties of nitrogen atoms in N2, which can help determine the hybridization and bonding characteristics of the molecule.
The understanding of the relationship between spectroscopic data and bond order of N2 has practical applications in various fields. For instance, in atmospheric chemistry, the bond order of N2 is important for understanding the stability and reactivity of nitrogen oxides, which play a role in air pollution and climate change. In materials science, the bond order of N2 is relevant for designing new materials with desired properties, such as high strength and thermal stability. In biochemistry, the bond order of N2 is crucial for understanding the nitrogen fixation process, which is essential for plant growth and food production.
Frequently Asked Questions about Bond Order of N2
This section addresses common questions and clarifies aspects related to calculating the bond order of nitrogen molecules (N2).
Question 1: What factors influence the bond order of N2?
Answer: Several factors affect the bond order of N2, including the number of valence electrons, hybridization of atomic orbitals, resonance, bond length, bond strength, and magnetic properties.
Question 2: How do I determine the bond order of N2 using molecular orbital theory?
Answer: Molecular orbital theory involves analyzing the combination of atomic orbitals to form molecular orbitals. The number of bonding and antibonding electrons in these molecular orbitals helps determine the bond order.
Question 3: What is the significance of resonance in calculating the bond order of N2?
Answer: Resonance structures contribute to the overall stability of N2 by delocalizing electrons. Considering resonance helps identify bonding and antibonding electrons, which is essential for accurately determining the bond order.
Question 4: How does hybridization affect the bond order of N2?
Answer: Hybridization involves mixing atomic orbitals to form hybrid orbitals with specific shapes. In N2, sp hybridization occurs, influencing the geometry and bonding characteristics, which ultimately affect the bond order.
Question 5: What experimental techniques provide insights into the bond order of N2?
Answer: Spectroscopic techniques such as UV-Vis, IR, and NMR spectroscopy offer valuable information about the electronic structure, vibrational modes, and nuclear spin properties of N2. This data aids in determining the bond order and understanding the chemical bonding characteristics.
Question 6: How is the bond order of N2 relevant to practical applications?
Answer: Understanding the bond order of N2 has implications in diverse fields. In atmospheric chemistry, it helps study nitrogen oxides’ stability and reactivity. In materials science, it guides the design of materials with desired properties. In biochemistry, it plays a role in comprehending nitrogen fixation, crucial for plant growth and food production.
These FAQs provide essential insights into calculating the bond order of N2. Further exploration of molecular orbital theory, hybridization, resonance, and experimental techniques can deepen the understanding of N2‘s bonding characteristics.
Transition to the next section: Delving into advanced concepts related to the bond order of N2, we will examine how these concepts extend our understanding of molecular bonding and its implications in various scientific disciplines.
Tips for Calculating Bond Order of N2
Understanding the nuances of calculating the bond order of nitrogen molecules (N2) is essential for comprehending their chemical bonding and behavior. Here are some practical tips to help you accurately determine the bond order of N2:
Tip 1: Begin by understanding the concept of molecular orbital theory and its application in describing the electronic structure of N2.
Tip 2: Determine the number of valence electrons in N2 and analyze their distribution in the molecular orbitals formed by the combination of atomic orbitals.
Tip 3: Identify the bonding and antibonding orbitals based on their symmetry and energy levels. The difference in the number of bonding and antibonding electrons contributes to the bond order.
Tip 4: Consider the hybridization of atomic orbitals involved in N2 bonding. This hybridization affects the geometry and orbital overlap, influencing the bond order.
Tip 5: Utilize experimental techniques such as UV-Vis, IR, and NMR spectroscopy to gather data on the electronic structure and bonding characteristics of N2. This data can support your bond order calculations.
Tip 6: Explore the concept of resonance and its implications for the bond order of N2. Resonance structures provide alternative representations of the electronic structure, contributing to the stability of the molecule.
Tip 7: Pay attention to the magnetic properties of N2, such as its diamagnetic nature and zero magnetic moment. These properties provide insights into the electronic configuration and bonding characteristics.
Tip 8: Familiarize yourself with the periodic trends and electronegativity values of nitrogen to understand how these factors influence the bond order of N2.
By following these tips, you can gain a comprehensive understanding of the factors that determine the bond order of N2 and apply this knowledge to various chemical contexts.
Transition to the conclusion: These tips provide a solid foundation for exploring the advanced concepts related to the bond order of N2. In the concluding section, we will delve deeper into the implications and applications of bond order calculations, solidifying our understanding of the chemical bonding in N2.
Conclusion
In this comprehensive analysis of “how to calculate bond order of N2“, we have explored the fundamental concepts, experimental techniques, and practical tips involved in determining the bond order of nitrogen molecules. Understanding the bond order of N2 provides valuable insights into its chemical bonding, stability, and reactivity.
Several key takeaways emerge from our exploration:
- The bond order of N2 is 3, indicating a strong and stable triple bond between the two nitrogen atoms.
- Molecular orbital theory, hybridization, and resonance play crucial roles in determining the bond order and electronic structure of N2.
- Experimental techniques such as UV-Vis, IR, and NMR spectroscopy provide valuable data for understanding the bonding characteristics and bond order of N2.
Beyond these core concepts, the study of bond order has far-reaching implications in diverse scientific disciplines. It enables researchers to design new materials with tailored properties, understand the behavior of nitrogen-containing compounds in atmospheric and environmental chemistry, and unravel the mechanisms of nitrogen fixation in biological systems.