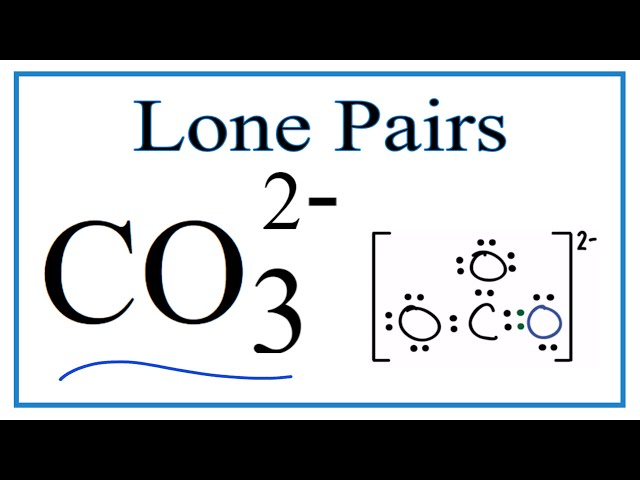
Bond order calculation is a fundamental aspect of understanding chemical bonding. In coordination chemistry, determining the bond order of carbonate ion (CO32-) is crucial for comprehending its structure, bonding, and reactivity.
Understanding bond order helps chemists predict the stability, reactivity, and electronic properties of molecules. The concept of bond order has played a significant role in the development of molecular orbital theory and computational chemistry.
This article will delve into the methods for calculating the bond order of carbonate ion, exploring the resonance structures, molecular orbital interactions, and experimental techniques used to determine its bonding characteristics.
How to Calculate Bond Order of Carbonate Ion
Calculating the bond order of carbonate ion is essential for understanding its chemical bonding and properties. Key aspects to consider include:
- Resonance structures
- Molecular orbital interactions
- Hybridization
- Electronegativity
- Bond length
- Bond strength
- IR spectroscopy
- X-ray crystallography
- Computational chemistry
These aspects provide insights into the nature of the carbonate ion’s bonding, its stability, and its reactivity. By understanding these aspects, chemists can better predict and explain the behavior of carbonate-containing compounds.
Resonance structures
Resonance structures are crucial for calculating the bond order of carbonate ion because they provide a way to represent the delocalization of electrons within the ion. Carbonate ion has three resonance structures, each of which contributes to the overall bonding and properties of the ion.
The three resonance structures of carbonate ion can be drawn by moving the double bond between the carbon and one of the oxygen atoms. This results in three equivalent structures, each with a single bond between the carbon and two of the oxygen atoms, and a double bond between the carbon and the remaining oxygen atom. The delocalization of electrons between these three resonance structures means that the carbon-oxygen bonds in carbonate ion have a bond order of 1.33, which is intermediate between a single and a double bond.
Understanding the resonance structures of carbonate ion is essential for accurately calculating its bond order and understanding its chemical bonding. Resonance structures also provide insights into the stability, reactivity, and spectroscopic properties of carbonate ion, making them a valuable tool for chemists.
Molecular orbital interactions
Molecular orbital interactions play a crucial role in determining the bond order of carbonate ion. By examining the interactions between the atomic orbitals of carbon and oxygen, we can gain insights into the electronic structure and bonding characteristics of the ion.
- Sigma interactions
Sigma interactions arise from the overlap of head-to-head atomic orbitals. In carbonate ion, the 2s orbital of carbon overlaps with the 2p orbitals of the three oxygen atoms, resulting in three sigma bonds.
- Pi interactions
Pi interactions stem from the overlap of parallel atomic orbitals. In carbonate ion, the 2p orbitals of carbon and oxygen overlap laterally, forming two pi bonds.
- Hybridization
Hybridization involves the mixing of atomic orbitals to form new hybrid orbitals with different shapes and energies. In carbonate ion, the carbon atom undergoes sp2 hybridization, resulting in three equivalent hybrid orbitals that form sigma bonds with the oxygen atoms.
- Resonance
Resonance occurs when multiple Lewis structures can be drawn for a molecule or ion. Carbonate ion has three resonance structures, which contribute to the delocalization of electrons and the equalization of bond lengths.
Understanding molecular orbital interactions is essential for accurately calculating the bond order of carbonate ion. These interactions dictate the electronic structure, bonding characteristics, and overall stability of the ion. By considering the various types of orbital interactions, hybridization, and resonance, we can gain a deeper understanding of the nature of the carbonate ion’s chemical bonding.
Hybridization
Hybridization plays a critical role in how to calculate the bond order of carbonate ion. Hybridization is the mixing of atomic orbitals to form new hybrid orbitals with different shapes and energies. In the case of the carbonate ion, the carbon atom undergoes sp2 hybridization. This means that the 2s orbital and two of the 2p orbitals of the carbon atom combine to form three equivalent hybrid orbitals. These hybrid orbitals then overlap with the p orbitals of the three oxygen atoms to form three sigma bonds.
The hybridization of the carbon atom in the carbonate ion has several important consequences. First, it explains the geometry of the ion. The three sigma bonds formed by the hybrid orbitals point in three directions, forming a trigonal planar geometry. Second, the hybridization affects the bond order of the carbonate ion. The bond order is a measure of the strength of the bond between two atoms, and it is calculated by dividing the number of bonding electrons by the number of bonds between the atoms. In the case of the carbonate ion, each carbon-oxygen bond has a bond order of 1.33. This is because the three sigma bonds are formed by the overlap of one bonding electron from each atom.
Understanding the hybridization of the carbon atom in the carbonate ion is essential for accurately calculating the bond order of the ion. The hybridization determines the geometry of the ion and the strength of the carbon-oxygen bonds. This information is important for understanding the chemical properties of the carbonate ion and its role in various chemical processes.
Electronegativity
Electronegativity plays a crucial role in how to calculate the bond order of carbonate ion. Electronegativity is a measure of the attraction of an atom for electrons. The greater the electronegativity of an atom, the more strongly it attracts electrons. In the case of the carbonate ion, the carbon atom is less electronegative than the oxygen atoms. This means that the oxygen atoms attract electrons more strongly than the carbon atom, resulting in a polarization of the electron cloud towards the oxygen atoms.
The polarization of the electron cloud in the carbonate ion has several important consequences. First, it affects the bond lengths between the carbon and oxygen atoms. The carbon-oxygen bonds are shorter than the typical C-O bond length. This is because the electron cloud is pulled towards the oxygen atoms, which reduces the distance between the carbon and oxygen atoms. Second, the polarization of the electron cloud affects the bond order of the carbonate ion. The bond order is a measure of the strength of the bond between two atoms. The bond order of the carbonate ion is less than 2, indicating that the carbon-oxygen bonds are not pure double bonds. This is because the electron cloud is not evenly distributed between the carbon and oxygen atoms.
Understanding the role of electronegativity in the carbonate ion is essential for accurately calculating the bond order of the ion. Electronegativity is a critical factor in determining the bond lengths, bond order, and overall geometry of the carbonate ion. This information is important for understanding the chemical properties of the carbonate ion and its role in various chemical processes.
Bond length
Bond length is the distance between two bonded atoms in a molecule. It is an important factor in determining the properties of a molecule, such as its stability, reactivity, and spectroscopic properties. Bond length is also closely related to bond order. In general, shorter bond lengths correspond to higher bond orders, and vice versa.
In the case of the carbonate ion, the bond length between the carbon and oxygen atoms is 1.22 . This is shorter than the typical C-O bond length of 1.43 . The shorter bond length in the carbonate ion is due to the higher bond order of the carbon-oxygen bonds. The carbon-oxygen bonds in the carbonate ion have a bond order of 1.33, which is between a single bond and a double bond. This is due to the resonance between the three resonance structures of the carbonate ion.
The bond length in the carbonate ion is a critical component of how to calculate bond order. By measuring the bond length, we can gain insights into the bond order and the electronic structure of the ion. This information is important for understanding the chemical properties of the carbonate ion and its role in various chemical processes.
Bond strength
In the context of calculating bond order for the carbonate ion (CO32-), bond strength plays a significant role in determining the stability and properties of the ion. Bond strength refers to the force that holds atoms together in a molecule or ion. Several factors contribute to bond strength, including:
- Bond length
Bond strength is inversely proportional to bond length. Shorter bonds are typically stronger than longer bonds because the atoms are held more closely together by the attractive forces between them.
- Bond order
Bond strength is directly proportional to bond order. Higher bond orders indicate a greater number of bonding electrons, which leads to a stronger bond.
- Electronegativity
The difference in electronegativity between two atoms affects bond strength. A greater difference in electronegativity leads to a more polar bond, which can be weaker than a nonpolar bond.
- Resonance
Resonance occurs when multiple Lewis structures can be drawn for a molecule or ion. Resonance can increase bond strength by delocalizing electrons and reducing the overall energy of the molecule.
Understanding bond strength is crucial for accurately calculating the bond order of the carbonate ion. By considering the factors that influence bond strength, chemists can gain insights into the stability and reactivity of the ion, as well as its role in various chemical processes.
IR spectroscopy
IR spectroscopy, a powerful analytical technique, finds extensive application in calculating the bond order of carbonate ion (CO32-). IR spectroscopy probes the vibrational modes of the carbonate ion, providing insights into its structure and bonding characteristics.
- Vibrational Modes
IR spectroscopy measures the absorption of infrared radiation by a sample, which corresponds to the excitation of vibrational modes within the molecule. Different bonds exhibit characteristic vibrational frequencies, allowing for the identification of functional groups and determination of bond order.
- IR-Active Bonds
Only bonds with a change in dipole moment during vibration are IR-active. The presence or absence of IR bands can provide information about the symmetry and bonding of the carbonate ion.
- Bond Strength
The strength of a bond is related to its vibrational frequency. Stronger bonds typically have higher vibrational frequencies. By measuring the IR absorption bands of the carbonate ion, the relative bond strengths of the carbon-oxygen bonds can be assessed.
- Resonance
The resonance between different Lewis structures of the carbonate ion can affect its IR spectrum. The presence of multiple resonance structures can lead to the splitting or broadening of IR bands, providing insights into the electronic structure of the ion.
The aforementioned facets of IR spectroscopy collectively contribute to the calculation of the bond order of the carbonate ion. By analyzing the IR spectrum of the ion, chemists can deduce information about its molecular structure, bonding characteristics, and resonance effects, enabling a comprehensive understanding of this important species.
X-ray crystallography
X-ray crystallography plays a crucial role in the determination of bond order for the carbonate ion (CO32-) by providing precise structural information at the atomic level.
- Crystal Structure
X-ray crystallography determines the arrangement of atoms within a crystal lattice, offering insights into the molecular geometry and bonding of the carbonate ion. By analyzing the diffraction patterns generated by X-rays interacting with the crystal, scientists can deduce the positions and orientations of individual atoms.
- Bond Lengths and Angles
X-ray crystallography provides accurate measurements of bond lengths and angles within the carbonate ion. These parameters are critical for calculating bond order, as they reflect the strength and nature of the chemical bonds. Shorter bond lengths and smaller bond angles typically indicate higher bond orders.
- Electron Density
X-ray crystallography can map the electron density distribution within the carbonate ion. This information helps determine the localization of electrons involved in bonding, providing insights into the electronic structure and resonance effects that influence bond order.
- Atomic Displacement Parameters
X-ray crystallography can reveal the thermal motion and disorder of atoms within the carbonate ion. These parameters affect the precision of bond order calculations and can provide information about the dynamic behavior of the ion.
By combining these facets, X-ray crystallography provides a wealth of structural data essential for accurately calculating the bond order of the carbonate ion. This information is crucial for understanding the chemical bonding and properties of this important species, contributing to a comprehensive understanding of its behavior in various chemical systems.
Computational chemistry
Computational chemistry plays a significant role in the calculation of bond order for the carbonate ion (CO32-), offering powerful tools to probe its electronic structure and bonding characteristics at the molecular level.
- Quantum mechanics
Quantum mechanics provides the theoretical foundation for computational chemistry, enabling the description and prediction of molecular properties based on the wave functions of electrons. By solving the Schrdinger equation for the carbonate ion, computational methods can determine its electronic structure, including the molecular orbitals and electron density distribution.
- Molecular modeling
Molecular modeling techniques, such as molecular dynamics and density functional theory (DFT), allow researchers to simulate the behavior of the carbonate ion in a virtual environment. These simulations can provide insights into the ion’s conformational changes, vibrational modes, and interactions with other molecules.
- Basis sets
Basis sets are sets of functions used to represent the molecular orbitals of the carbonate ion. The choice of basis set can significantly affect the accuracy of computational results. Common basis sets include Gaussian-type orbitals and Slater-type orbitals.
- Software applications
Computational chemistry software, such as Gaussian, ORCA, and NWChem, provide user-friendly interfaces and powerful algorithms for performing quantum mechanical calculations. These software packages enable researchers to model and analyze the carbonate ion’s electronic structure, optimize its geometry, and compute its various properties.
The integration of these computational chemistry facets allows scientists to gain deep insights into the bonding nature of the carbonate ion. By leveraging the power of computational methods, researchers can accurately calculate bond orders, predict molecular properties, and explore the reactivity and behavior of this important species in diverse chemical environments.
Frequently Asked Questions
The following FAQs provide concise answers to common questions and clarify key concepts related to calculating the bond order of carbonate ion.
Question 1: What is bond order and why is it important?
Answer: Bond order is a measure of the strength of a chemical bond and is calculated based on the number of bonding electrons. Understanding bond order helps predict the stability, reactivity, and electronic properties of molecules.
Question 2: How do I calculate the bond order of carbonate ion using resonance structures?
Answer: Carbonate ion has three resonance structures. The bond order is calculated as the average number of bonds between the carbon and oxygen atoms across all resonance structures.
Question 3: What role does hybridization play in determining bond order?
Answer: Hybridization involves mixing atomic orbitals to form new hybrid orbitals with different shapes and energies. The type of hybridization affects the geometry and bond order of the molecule.
Question 4: How can I use experimental techniques to determine bond order?
Answer: Experimental techniques such as IR spectroscopy, X-ray crystallography, and computational chemistry provide valuable data on bond lengths, vibrational frequencies, and electronic structures, which can be used to calculate bond order.
Question 5: What are the limitations of using theoretical methods to calculate bond order?
Answer: Theoretical methods rely on approximations and assumptions, which can introduce some level of uncertainty into the calculated bond order values.
Question 6: How does bond order impact the chemical properties of carbonate ion?
Answer: Bond order influences various properties, including bond strength, reactivity, and molecular stability. Understanding bond order provides insights into the behavior and applications of carbonate ion in different chemical systems.
These FAQs offer fundamental insights into the concept of bond order and its calculation for carbonate ion. In the next section, we will explore advanced topics related to bond order and its implications for the chemistry of carbonate compounds.
Tips for Calculating Bond Order of Carbonate Ion
To enhance your understanding and accuracy when calculating the bond order of carbonate ion, consider the following practical tips:
Tip 1: Identify resonance structures. Understand the different resonance structures of carbonate ion and their contribution to the overall bonding.
Tip 2: Examine molecular orbital interactions. Analyze the interactions between atomic orbitals to determine the type and strength of bonds formed.
Tip 3: Consider hybridization. Determine the hybridization of the carbon atom to understand the geometry and bond order of the ion.
Tip 4: Evaluate electronegativity. Assess the electronegativity difference between carbon and oxygen atoms, as it affects bond polarity and bond order.
Tip 5: Measure bond length. Utilize experimental techniques like X-ray crystallography to obtain precise bond length data, which correlates with bond order.
Tip 6: Analyze bond strength. Study the factors influencing bond strength, such as bond length and resonance, to gain insights into the stability of the carbonate ion.
Tip 7: Employ IR spectroscopy. Use IR spectroscopy to identify vibrational modes and determine bond order based on the frequencies of absorption bands.
Tip 8: Leverage computational chemistry. Utilize computational methods like DFT to model and calculate the bond order of carbonate ion, considering electron density distribution and molecular orbitals.
By incorporating these tips into your approach, you will enhance the accuracy and depth of your understanding regarding the bond order of carbonate ion.
In the concluding section, we will delve into the broader implications of bond order for the chemistry of carbonate compounds, exploring its significance in various applications and research areas.
Conclusion
In summary, this article has provided a comprehensive exploration of how to calculate the bond order of carbonate ion. We have examined various methods for calculating bond order, including resonance structures, molecular orbital interactions, and experimental techniques. Our analysis has highlighted the importance of considering hybridization, electronegativity, and bond strength in determining the bond order of carbonate ion.
The concept of bond order serves as a fundamental tool for understanding the chemical bonding and properties of carbonate compounds. By accurately calculating bond order, chemists can gain insights into the stability, reactivity, and behavior of these important species. Moreover, the methods described in this article can be applied to a wide range of other molecules and ions, providing a valuable approach for investigating chemical bonding and molecular structure.