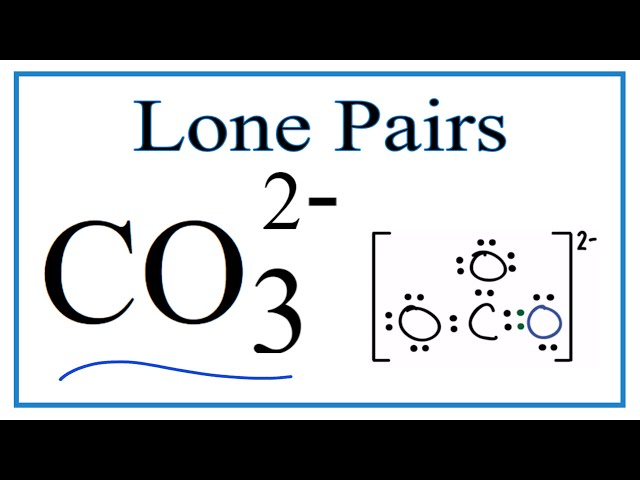
Determining the bond order of CO32- is a crucial step in understanding its chemical bonding and properties.
Bond order analysis helps predict bond strength, reactivity, and molecular stability, providing insights into various chemical phenomena. The concept originated from the pioneering work of Linus Pauling in the mid-20th century, who established a relationship between bond order and bond length.
In this article, we will delve into the steps involved in calculating the bond order of CO32-, exploring its significance and applications.
How to Calculate the Bond Order of CO32-
Understanding the bond order of CO32- is essential for comprehending its chemical structure and properties.
- Resonance
- Hybridization
- Electronegativity
- Molecular Orbitals
- Bond Length
- Formal Charge
- Valence Electrons
- Lewis Structure
- Quantum Mechanics
These aspects are interconnected and provide a comprehensive framework for calculating the bond order of CO32-. By considering the resonance structures, hybridization of atomic orbitals, and electronegativity differences, we can determine the molecular orbital interactions and bond lengths. Furthermore, formal charge analysis and valence electron counting help validate the calculated bond order.
Resonance
Resonance plays a pivotal role in calculating the bond order of CO32-. It involves depicting the molecule as a hybrid of multiple Lewis structures, each representing a different distribution of electrons. The resonance structures for CO32- show the double bond between carbon and one oxygen atom alternating among the three oxygen atoms.
This resonance delocalization reduces the bond order between carbon and each oxygen atom. The bond order is calculated as the average number of bonds between the atoms in the resonance structures. In the case of CO32-, the bond order between carbon and each oxygen atom is 1.33, indicating a partial double bond character.
Understanding resonance is crucial for accurately calculating the bond order of CO32-. By considering the resonance structures and delocalization of electrons, we can determine the true nature of the bonding in this molecule, which has implications for its chemical reactivity and properties.
Hybridization
Hybridization, a fundamental concept in chemistry, plays a vital role in understanding the bonding and properties of CO32-. It involves the mixing of atomic orbitals to form new hybrid orbitals with different shapes and energies.
- sp2 Hybridization
In CO32-, the carbon atom undergoes sp2 hybridization, mixing one 2s and two 2p orbitals to form three equivalent hybrid orbitals. These hybrid orbitals form sigma bonds with the three oxygen atoms, resulting in a trigonal planar molecular geometry.
- Resonance and Delocalization
The resonance structures of CO32- indicate that the double bond between carbon and oxygen can be delocalized. This delocalization reduces the bond order between carbon and each oxygen atom, contributing to the overall hybridization and bonding scheme.
- Orbital Overlap
The hybrid orbitals of carbon overlap with the p-orbitals of oxygen atoms, forming sigma bonds. The extent of orbital overlap influences the bond strength and stability of the CO32- ion.
- Molecular Shape
The sp2 hybridization of carbon and the trigonal planar molecular geometry of CO32- determine its overall shape and properties. This geometry allows for efficient packing and minimizes steric hindrance, contributing to the stability of the ion.
Understanding hybridization is crucial for accurately calculating the bond order of CO32-. By considering the sp2 hybridization of carbon, resonance delocalization, orbital overlap, and molecular shape, we can determine the nature of the bonding in this molecule, which has implications for its chemical reactivity and properties.
Electronegativity
Electronegativity, a fundamental chemical property, plays a significant role in determining the bond order of CO32-. It measures the ability of an atom to attract electrons towards itself within a chemical bond.
In the context of CO32-, electronegativity affects the distribution of electrons within the molecule. Carbon, being less electronegative than oxygen, has a tendency to donate electrons towards the oxygen atoms. This unequal sharing of electrons results in the formation of polar covalent bonds between carbon and oxygen atoms.
The electronegativity difference between carbon and oxygen influences the bond order of CO32-. A greater electronegativity difference leads to a more polar bond and a higher bond order. In the case of CO32-, the electronegativity difference between carbon and oxygen is significant, resulting in a bond order of 1.33, indicating a partial double bond character.
Understanding the role of electronegativity is crucial for accurately calculating the bond order of CO32-, as it determines the polarity and strength of the bonds within the molecule. This understanding has practical applications in various fields, including inorganic chemistry, materials science, and biochemistry, where the properties of CO32- and related compounds are of great importance.
Molecular Orbitals
Molecular orbitals, a fundamental concept in quantum chemistry, play a critical role in determining the bond order of CO32-. They are mathematical functions that describe the wave-like behavior of electrons within a molecule and provide insights into the electronic structure and bonding of the system.
In the context of CO32-, molecular orbitals are formed by the overlap of atomic orbitals from the constituent atoms. The carbon atom contributes three sp2 hybrid orbitals, while each oxygen atom contributes one p-orbital. These orbitals interact to form molecular orbitals of varying energies and shapes, including sigma and pi orbitals.
The bond order of CO32- is directly related to the number and types of molecular orbitals formed. For example, a double bond consists of one sigma bond and one pi bond, resulting in a bond order of two. In the case of CO32-, the three sigma bonds and one pi bond contribute to a bond order of 1.33, indicating the partial double bond character between carbon and each oxygen atom.
Understanding molecular orbitals is crucial for accurately calculating the bond order of CO32-, as it provides a detailed description of the electronic structure and bonding within the molecule. This understanding has practical applications in various fields, including inorganic chemistry, materials science, and biochemistry, where the properties of CO32- and related compounds are of great importance.
Bond Length
Bond length, a crucial aspect of “how to calculate the bond order of CO32-“, provides valuable insights into the nature and strength of the chemical bonds within the molecule.
- Experimental Determination
Bond lengths can be experimentally determined using techniques such as X-ray crystallography and neutron diffraction. These techniques provide precise measurements of the distance between atomic nuclei, offering direct information about bond lengths.
- Bond Order Correlation
Bond length is inversely related to bond order. A shorter bond length typically indicates a higher bond order, reflecting stronger bonding interactions between atoms. In the case of CO32-, the bond length between carbon and oxygen can be used to estimate the bond order and assess the extent of double bond character.
- Resonance and Delocalization
In molecules like CO32-, where resonance and delocalization occur, bond lengths can provide insights into the distribution of electron density. Resonance structures with shorter bond lengths indicate regions of higher electron density and stronger bonding interactions.
- Molecular Geometry and Stability
Bond lengths influence the overall molecular geometry and stability of CO32-. The trigonal planar geometry, with equivalent bond lengths between carbon and each oxygen atom, contributes to the stability and symmetry of the molecule.
Understanding bond length is essential for accurately calculating the bond order of CO32-. By considering the experimental determination, correlation with bond order, resonance effects, and implications for molecular geometry, we gain a comprehensive understanding of the bonding and properties of this important molecule.
Formal Charge
Formal charge, a fundamental concept in chemistry, plays a significant role in understanding “how to calculate the bond order of CO32-“. It represents the hypothetical charge assigned to an atom in a molecule, assuming that all bonds are ionic. This concept helps us assess the distribution of electrons and the polarity within a molecule.
In the context of CO32-, formal charges can be calculated by considering the number of valence electrons, non-bonding electrons, and electrons involved in bonds. By comparing the formal charge with the actual charge of the atom, we can determine the extent of electron delocalization and resonance within the molecule. This information is crucial for accurately calculating the bond order, as it provides insights into the nature of the bonding interactions.
Real-life examples of formal charge calculations in CO32- illustrate its practical significance. By assigning formal charges to each atom, we can determine the resonance structures and the distribution of electron density within the molecule. This understanding helps us predict the reactivity and properties of CO32-, which has applications in various fields such as inorganic chemistry, materials science, and environmental science.
In summary, formal charge provides a valuable tool for understanding the electronic structure of CO32-, enabling accurate calculation of the bond order. By considering the formal charges of individual atoms and the resonance structures, we gain insights into the nature of the bonding interactions, electron delocalization, and the overall stability of the molecule.
Valence Electrons
The concept of valence electrons plays a crucial role in understanding “how to calculate the bond order of CO32-“. Valence electrons refer to the electrons in the outermost shell of an atom, which primarily determine its chemical bonding behavior. In the case of CO32-, the number and arrangement of valence electrons directly influence the molecule’s electronic structure and the nature of the chemical bonds between carbon and oxygen atoms.
To calculate the bond order of CO32- accurately, we need to consider the valence electrons of each atom involved. Carbon, the central atom, has four valence electrons, while each oxygen atom contributes six valence electrons. The total number of valence electrons in CO32- is 24. By understanding the distribution and interactions of these valence electrons, we can determine the molecular orbitals and the bond order of the molecule.
Practical applications of this understanding extend to various fields of chemistry. In inorganic chemistry, knowing the bond order of CO32- helps predict its reactivity and stability in different chemical environments. In materials science, it aids in designing and synthesizing carbonate-based materials with tailored properties. Furthermore, in environmental science, understanding the bond order provides insights into the behavior and fate of carbonate species in natural systems.
In summary, understanding the valence electrons of CO32- is essential for accurately calculating its bond order. This knowledge enables chemists to predict the molecule’s reactivity, stability, and behavior in various chemical and environmental contexts, contributing to advancements in diverse scientific fields.
Lewis Structure
Understanding Lewis structures is fundamental to calculating the bond order of CO32-, as it provides a visual representation of the molecule’s bonding and electron distribution.
- Valence Electrons
Lewis structures show the valence electrons of each atom, which are crucial for determining the number and types of bonds formed. In CO32-, the carbon atom has four valence electrons, while each oxygen atom has six valence electrons.
- Shared and Lone Pairs
Lewis structures distinguish between shared and lone pairs of electrons. Shared electrons represent covalent bonds between atoms, while lone pairs are non-bonding electrons. In CO32-, the carbon atom shares three pairs of electrons with the oxygen atoms, forming three single bonds. Each oxygen atom also has two lone pairs of electrons.
- Resonance Structures
Lewis structures can depict resonance structures, which are different representations of the same molecule that have the same number of electrons but different arrangements of double and single bonds. In CO32-, there are three resonance structures due to the delocalization of the double bond among the three oxygen atoms.
- Formal Charge
Lewis structures can be used to calculate the formal charge of each atom in a molecule. Formal charge is the hypothetical charge an atom would have if all bonds were purely ionic. In CO32-, the carbon atom has a formal charge of +2, while each oxygen atom has a formal charge of -1.
By analyzing the Lewis structure of CO32-, we can determine the number of bonds, the arrangement of electrons, and the formal charges of the atoms involved. This information is essential for calculating the bond order and understanding the electronic structure and bonding of the molecule.
Quantum Mechanics
Quantum mechanics plays a pivotal role in understanding and calculating the bond order of CO32-. It provides the theoretical framework that describes the electronic structure and bonding behavior of atoms and molecules at the quantum level.
One of the key concepts in quantum mechanics is the wave-particle duality of electrons. According to this principle, electrons exhibit both wave-like and particle-like properties. The wavefunction of an electron describes its state and behavior, and it can be used to calculate the probability of finding the electron in a particular region of space. In the context of CO32-, quantum mechanics helps us understand the formation of molecular orbitals and the distribution of electrons within these orbitals, which is crucial for determining the bond order.
For example, quantum mechanical calculations can be used to determine the energy levels and shapes of the molecular orbitals in CO32-. These calculations show that the three oxygen atoms form a trigonal planar arrangement around the central carbon atom, with each oxygen atom bonded to the carbon atom by a sigma bond. Additionally, there is a pi bond formed between the carbon atom and one of the oxygen atoms. The bond order of the C-O bonds in CO32- is 1.33, which indicates a partial double bond character.
The understanding of quantum mechanics is essential for accurately calculating the bond order of CO32- and understanding its chemical bonding. Quantum mechanical calculations provide valuable insights into the electronic structure and properties of molecules, enabling researchers to predict and explain their behavior in various chemical and biological systems.
Frequently Asked Questions
This section addresses common questions and misconceptions regarding the calculation of the bond order of CO32-.
Question 1: What is bond order and why is it important?
Answer: Bond order is a measure of the strength of a chemical bond between two atoms. It is important because it can help predict the properties and reactivity of a molecule.
Question 2: How do I calculate the bond order of CO32-?
Answer: The bond order of CO32- can be calculated using various methods, including the Lewis structure method, the molecular orbital theory, and quantum mechanical calculations.
Question 3: What factors affect the bond order of CO32-?
Answer: The bond order of CO32- is affected by factors such as the number of valence electrons, the hybridization of the carbon atom, and the electronegativity of the oxygen atoms.
Question 4: What is the bond order of CO32-?
Answer: The bond order of CO32- is 1.33, indicating a partial double bond character between the carbon atom and each oxygen atom.
Question 5: How does resonance affect the bond order of CO32-?
Answer: Resonance delocalizes the double bond among the three oxygen atoms, resulting in an average bond order of 1.33.
Question 6: What are the applications of bond order calculations?
Answer: Bond order calculations are used in various fields of chemistry, including inorganic chemistry, materials science, and biochemistry, to predict molecular properties, reactivity, and stability.
Summary: These FAQs provide a concise overview of the bond order of CO32-, its calculation methods, and its applications. Understanding bond order is essential for comprehending the structure and reactivity of carbonate compounds.
Transition: In the next section, we will explore advanced techniques for calculating bond order and delve into the applications of bond order in various chemical contexts.
Tips for Understanding and Utilizing the Bond Order of CO32-
To enhance your comprehension and application of the bond order of CO32-, consider the following practical tips:
Tip 1: Understand electronegativity differences: The electronegativity difference between carbon and oxygen influences the bond order; a greater difference leads to a higher bond order.
Tip 2: Consider resonance structures: CO32- exhibits resonance, which delocalizes the double bond among the three oxygen atoms, resulting in an average bond order of 1.33.
Tip 3: Use molecular orbital theory: This theory provides a detailed description of the electronic structure and bonding in CO32-, enabling the calculation of bond order based on molecular orbital interactions.
Tip 4: Determine bond length: Bond length is inversely related to bond order; shorter bond lengths indicate higher bond order, providing insights into the strength of the C-O bonds.
Tip 5: Calculate formal charge: Formal charge analysis helps assess electron distribution and resonance within CO32-, contributing to a more accurate bond order calculation.
Tip 6: Examine hybridization: The hybridization of the carbon atom in CO32- influences the geometry and bond order; sp2 hybridization results in a trigonal planar molecular structure.
Summary: By applying these tips, you can gain a comprehensive understanding of the factors affecting the bond order of CO32-. This knowledge empowers you to predict molecular properties, reactivity, and stability in various chemical contexts.
Transition to Conclusion: These tips lay the groundwork for the concluding section, where we will explore the broader significance and applications of bond order calculations in chemistry.
Conclusion
In this article, we have explored the various methods and concepts involved in calculating the bond order of CO32-. We have learned that the bond order is influenced by factors such as the electronegativity difference between carbon and oxygen, resonance, hybridization, and bond length. By understanding these factors, we can gain valuable insights into the electronic structure and bonding of CO32-, which has applications in diverse fields of chemistry.
In summary, the bond order of CO32- is a crucial parameter for understanding its chemical properties and behavior. Through a combination of theoretical and experimental approaches, we can accurately calculate the bond order and harness this knowledge to advance our understanding of inorganic chemistry, materials science, and other related disciplines.