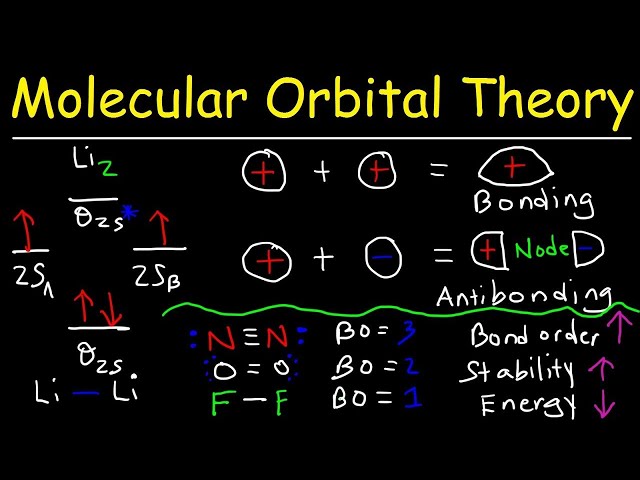
A bond order molecular orbital, a quantitative measure of the strength of a covalent bond, reflects the number of electrons occupying the molecular orbital formed by the overlap of atomic orbitals. In chemistry, determining bond order holds immense significance. It provides insights into bond properties, chemical reactivity, and molecular stability. For instance, in carbon monoxide (CO), the bond order of 3 indicates a strong covalent bond between carbon and oxygen, contributing to its stability and unique reactivity.
Understanding bond order has been instrumental in advancing chemical theory and applications. The concept originated from the pioneering work of Linus Pauling in the 1930s, who developed quantum mechanical models to explain chemical bonding. Pauling’s contributions laid the foundation for our current understanding of molecular orbital theory and its applications in various fields, including materials science and biochemistry.
This article delves into the intricacies of finding bond order molecular orbitals, exploring the theoretical framework, practical methods, and implications for chemical understanding and applications.
How to Find Bond Order Molecular Orbital
Understanding the key aspects of bond order molecular orbitals is crucial for comprehending chemical bonding and molecular properties.
- Atomic Orbital Overlap
- Electron Density
- Bond Strength
- Molecular Geometry
- Quantum Mechanics
- Molecular Orbital Theory
- Hybridization
- Resonance
- Delocalization
These aspects are interconnected and provide a comprehensive framework for determining bond order molecular orbitals. Quantum mechanics and molecular orbital theory provide the theoretical foundation, while atomic orbital overlap and electron densityare key factors in bond formation. Bond strength and molecular geometry are directly influenced by bond order, and concepts like hybridization, resonance, and delocalization further refine our understanding of molecular bonding. By exploring these aspects, we gain insights into the electronic structure and behavior of molecules.
Atomic Orbital Overlap
Atomic orbital overlap is a fundamental concept in understanding how to find bond order molecular orbitals. It refers to the extent to which atomic orbitals on adjacent atoms overlap, creating a region of electron density that constitutes a chemical bond. The degree of overlap directly influences the strength and properties of the bond.
In the context of bond order molecular orbitals, atomic orbital overlap plays a critical role in determining the number and type of molecular orbitals formed. Greater overlap leads to stronger interactions and the formation of lower-energy molecular orbitals. For instance, in the case of a sigma bond, head-to-head overlap of atomic orbitals results in a bonding molecular orbital with lower energy than the original atomic orbitals. Conversely, less overlap results in weaker interactions and higher-energy molecular orbitals.
Real-life examples of atomic orbital overlap can be observed in various molecules. In the water molecule (H2O), the overlap of the 1s orbital of each hydrogen atom with the 2p orbitals of the oxygen atom forms the O-H sigma bonds. The extent of overlap determines the bond length and strength, which are crucial for understanding the molecular geometry and reactivity of water. Similarly, in carbon dioxide (CO2), the overlap of the 2p orbitals of the carbon atom with the 2p orbitals of the oxygen atoms forms the C-O double bonds. The degree of overlap influences the bond order, which in turn affects the stability and reactivity of the molecule.
Understanding atomic orbital overlap is essential for predicting and interpreting various chemical phenomena. It enables chemists to rationalize bond strengths, molecular geometries, and chemical reactivity. This understanding finds practical applications in diverse fields, including materials science, catalysis, and drug design. By manipulating atomic orbital overlap through techniques like hybridization and resonance, scientists can design and synthesize materials with tailored properties and functionalities.
Electron Density
Electron density, a fundamental property of molecules, plays a crucial role in finding bond order molecular orbitals. It refers to the distribution of electrons in space around the atomic nuclei. Understanding electron density is essential for comprehending chemical bonding and molecular behavior.
- Total Electron Density: Represents the overall electron distribution in a molecule, providing insights into molecular shape and size.
- Bonding Electron Density: Concentrated in the regions between atomic nuclei where chemical bonds form, indicating the strength and type of bonding interactions.
- Lone Pair Electron Density: Associated with non-bonding electrons, influencing molecular geometry and reactivity.
- Delocalized Electron Density: Spread over a larger region, delocalized electrons contribute to resonance and molecular stability.
Electron density analysis provides valuable information about bond order molecular orbitals. By examining the distribution of electrons, chemists can predict and interpret various chemical phenomena. For instance, regions of high electron density indicate strong bonding interactions, while areas of low electron density suggest weaker bonds or non-bonding interactions. This understanding enables the determination of bond orders, which are essential for comprehending molecular stability, reactivity, and properties.
Bond Strength
Bond strength, a measure of the force required to break a chemical bond, is intricately connected to the concept of bond order molecular orbitals. Understanding this relationship is crucial for comprehending the stability and reactivity of molecules.
Bond strength is directly proportional to bond order. Higher bond orders indicate stronger bonds, while lower bond orders correspond to weaker bonds. This relationship arises from the number of electrons occupying the bonding molecular orbitals. In a stronger bond, more electrons are present in the bonding orbitals, leading to a greater overlap between atomic orbitals and a stronger electrostatic attraction between the nuclei and electrons. Conversely, weaker bonds have fewer electrons in the bonding orbitals, resulting in less overlap and a weaker attraction.
For instance, in the case of carbon-carbon bonds, a single bond has a bond order of 1, a double bond has a bond order of 2, and a triple bond has a bond order of 3. The bond strength follows the same trend, with the triple bond being the strongest and the single bond being the weakest. This understanding is essential in predicting the stability and reactivity of various carbon-based molecules.
Determining bond order molecular orbitals is critical for understanding bond strength because it provides insights into the electronic structure and bonding interactions within a molecule. By calculating the bond order, chemists can make informed predictions about the strength and properties of chemical bonds, enabling them to design and synthesize materials with tailored functionalities and properties.
In summary, bond strength and bond order molecular orbitals are closely related concepts that provide valuable insights into the nature of chemical bonds. Understanding this relationship is fundamental for comprehending molecular stability, reactivity, and properties, with practical applications in diverse fields such as materials science, catalysis, and drug design.
Molecular Geometry
Molecular geometry, the arrangement of atoms in space, is inextricably linked to the determination of bond order molecular orbitals. It provides valuable insights into the electronic structure and properties of molecules, enabling chemists to understand and predict their behavior.
Molecular geometry is a consequence of the electron density distribution around atoms. The repulsion between electrons and the attraction between electrons and nuclei dictate the spatial arrangement of atoms to minimize energy. This arrangement influences the overlap of atomic orbitals, which in turn affects the formation and properties of molecular orbitals. By understanding molecular geometry, chemists can infer the number and type of bonds formed, as well as their relative strengths.
For instance, consider the water molecule (H2O). Its bent geometry results from the repulsion between the lone pairs of electrons on the oxygen atom. This geometry influences the overlap of atomic orbitals, leading to the formation of two O-H sigma bonds with a bond order of 1. In contrast, carbon dioxide (CO2) adopts a linear geometry due to the absence of lone pairs on the central carbon atom. This geometry allows for maximum overlap of atomic orbitals, resulting in two C-O double bonds with a bond order of 2.
Understanding the relationship between molecular geometry and bond order molecular orbitals has practical applications in various fields. In materials science, it guides the design of materials with specific properties by manipulating molecular geometry to achieve desired bond strengths and arrangements. In drug design, it aids in understanding the interactions between drugs and biological targets, enabling the development of more effective and targeted therapies. Furthermore, this understanding is essential for comprehending chemical reactivity, catalysis, and molecular spectroscopy.
In summary, molecular geometry is a critical component of finding bond order molecular orbitals. It provides insights into the electronic structure and properties of molecules, enabling chemists to understand and predict their behavior. This understanding has far-reaching applications in diverse fields, contributing to the advancement of scientific research and technological innovations.
Quantum Mechanics
Quantum mechanics underpins our understanding of bond order molecular orbitals by providing a theoretical framework that describes the behavior of electrons at the atomic and molecular level. It offers a set of principles and mathematical tools to calculate and predict the properties of molecules, including their electronic structure and bonding interactions.
- Wave-Particle Duality: Electrons exhibit both wave-like and particle-like properties. This duality affects the formation of molecular orbitals, as electrons can be delocalized over a region of space, influencing bond order and molecular properties.
- Uncertainty Principle: The position and momentum of an electron cannot be simultaneously determined with absolute precision. This uncertainty principle influences the description of electron density and the shape of molecular orbitals, impacting bond order calculations.
- Quantization of Energy: Electrons occupy discrete energy levels within atoms and molecules. The energy of these levels is quantized, meaning it can only take on specific values. This quantization affects the formation and properties of molecular orbitals and influences bond order determination.
- Superposition: Electrons can exist in multiple states simultaneously. This superposition principle contributes to the formation of hybrid orbitals and the delocalization of electrons, influencing bond order and molecular properties.
These facets of quantum mechanics collectively provide a powerful framework for understanding and predicting the behavior of electrons in molecules. By incorporating quantum mechanical principles into the determination of bond order molecular orbitals, chemists can gain deeper insights into the electronic structure, bonding, and properties of various chemical systems.
Molecular Orbital Theory
Molecular orbital theory is a fundamental pillar in the quest to find bond order molecular orbitals. It provides a powerful framework for understanding the electronic structure and bonding in molecules, enabling the calculation and prediction of bond orders.
- Linear Combination of Atomic Orbitals (LCAO): Molecular orbitals are constructed as linear combinations of atomic orbitals, providing a mathematical basis for describing electron density distribution and bond formation.
- Aufbau Principle: Electrons fill molecular orbitals in the order of increasing energy, following the same rules as atomic orbitals, guiding the population of bonding and antibonding orbitals.
- Hund’s Rule: When multiple degenerate molecular orbitals are available, electrons occupy them singly with parallel spins, maximizing the overall spin multiplicity, influencing bond order and magnetic properties.
- Molecular Orbital Diagrams: Visual representations of molecular orbitals, showing their energy levels, shapes, and electron occupation, providing insights into bond order, bonding interactions, and molecular properties.
These facets of molecular orbital theory collectively provide a comprehensive framework for determining bond order molecular orbitals. By considering the interactions, energies, and occupancies of molecular orbitals, chemists can gain a deeper understanding of the electronic structure and bonding in molecules, enabling the prediction and interpretation of various chemical phenomena.
Hybridization
In the quest to understand how to find bond order molecular orbitals, hybridization plays a pivotal role. It involves the intermixing of atomic orbitals to form new hybrid orbitals with different shapes and energies, offering insights into molecular bonding and properties.
- sp Hybridization: Two atomic orbitals, one s and one p, combine to form two equivalent sp hybrid orbitals, oriented 180 apart. This hybridization is observed in molecules like BeF2 and CH2O, influencing their linear and trigonal planar geometries, respectively.
- sp2 Hybridization: One s and two p orbitals hybridize to form three equivalent sp2 hybrid orbitals, arranged in a trigonal planar geometry. Molecules with sp2 hybridization, such as BF3 and CO2, exhibit trigonal planar and linear shapes, respectively.
- sp3 Hybridization: Hybridization involving one s and three p orbitals results in four equivalent sp3 hybrid orbitals, directed towards the corners of a tetrahedron. This is common in molecules like CH4 and NH3, leading to their tetrahedral and trigonal pyramidal geometries, respectively.
- d2sp3 Hybridization: In certain transition metal complexes, d orbitals participate in hybridization along with s and p orbitals. This hybridization gives rise to octahedral or square planar molecular geometries, influencing the coordination chemistry and properties of these complexes.
Understanding hybridization is crucial for finding bond order molecular orbitals, as it provides insights into the shapes, orientations, and energies of the orbitals involved in bonding. This knowledge enables chemists to predict molecular geometries, bond strengths, and various chemical properties, paving the way for the design and synthesis of molecules with tailored functionalities.
Resonance
Resonance is a fundamental concept in chemistry that provides a deeper understanding of the electronic structure and bonding in molecules. It is particularly relevant in the context of finding bond order molecular orbitals, as it offers insights into the delocalization of electrons and the resonance hybrid that accurately describes the molecular system.
In resonance, a molecule or polyatomic ion can be represented by multiple Lewis structures, each depicting a different arrangement of electrons. These structures are called resonance contributors, and they contribute to the overall resonance hybrid, which is a weighted average of the contributors. The resonance hybrid provides a more accurate representation of the molecule’s electronic structure than any single Lewis structure.
A classic example of resonance is benzene, a six-membered ring of carbon atoms with alternating single and double bonds. The Kekule structures of benzene, which show alternating single and double bonds, are two resonance contributors. However, the resonance hybrid of benzene depicts a uniform distribution of electrons around the ring, with bond lengths that are intermediate between single and double bonds. This delocalization of electrons contributes to the stability and unique properties of benzene.
Understanding resonance is critical for finding bond order molecular orbitals because it provides insights into the delocalization of electrons and the resonance hybrid. By considering the resonance contributors and the resonance hybrid, chemists can gain a more accurate picture of the molecular orbitals and their occupancies. This understanding is essential for predicting molecular properties, such as bond lengths, bond strengths, and chemical reactivity.
Delocalization
Delocalization, a fundamental concept in chemistry, describes the spreading or distribution of electrons over multiple atoms or molecular orbitals. Delocalization is prevalent in various molecules and significantly influences their properties, including their bond order molecular orbitals.
In the context of finding bond order molecular orbitals, delocalization plays a critical role. Delocalized electrons are not confined to a specific bond or atom but are spread over a larger region, affecting the overall electronic structure of the molecule. This delocalization can lead to the formation of resonance hybrids, where multiple contributing structures are used to describe the molecule’s electronic structure. By considering delocalization, chemists can gain insights into the resonance and hybridization of molecular orbitals, providing a more accurate representation of the molecule’s bonding and properties.
A classic example of delocalization within bond order molecular orbitals is benzene. The Kekule structures of benzene depict alternating single and double bonds, but the actual electronic structure involves the delocalization of electrons around the ring. This delocalization results in a resonance hybrid where all carbon-carbon bonds have the same bond order, intermediate between a single and double bond. Understanding this delocalization is essential for accurately determining the bond order molecular orbitals of benzene and explaining its unique stability and chemical properties.
The concept of delocalization not only aids in finding bond order molecular orbitals but also has wide-ranging practical applications. Delocalized electrons can enhance molecular stability, influence chemical reactivity, and contribute to the development of novel materials. For example, delocalization in conjugated polymers gives rise to their electrical conductivity, making them promising candidates for organic electronics and optoelectronics.
Frequently Asked Questions
This section provides answers to common questions and clarifies certain aspects related to finding bond order molecular orbitals.
Question 1: What is the significance of bond order molecular orbitals?
Answer: Bond order molecular orbitals provide valuable insights into the strength, properties, and reactivity of chemical bonds. They help us understand the electronic structure of molecules and predict their behavior.
Question 2: How do I determine the bond order of a molecular orbital?
Answer: The bond order of a molecular orbital is calculated based on the number of electrons occupying it. Higher bond orders indicate stronger bonds, while lower bond orders suggest weaker bonds.
Question 3: What factors influence the bond order of molecular orbitals?
Answer: The bond order is influenced by atomic orbital overlap, electron density, and the number of electrons in the molecular orbital.
Question 4: How does hybridization affect bond order molecular orbitals?
Answer: Hybridization involves the mixing of atomic orbitals to form new hybrid orbitals with different shapes and energies. This can influence the overlap of atomic orbitals and, consequently, the bond order molecular orbitals.
Question 5: What is the role of resonance in bond order molecular orbitals?
Answer: Resonance occurs when a molecule or ion can be represented by multiple Lewis structures. This delocalization of electrons can affect the bond order molecular orbitals and provide a more accurate description of the molecule’s electronic structure.
Question 6: How can I apply the concept of bond order molecular orbitals in practical applications?
Answer: Understanding bond order molecular orbitals is essential for predicting molecular properties, such as bond lengths, bond strengths, and chemical reactivity. This knowledge finds applications in various fields, including materials science, catalysis, and drug design.
These FAQs provide a concise overview of key concepts related to bond order molecular orbitals. In the following sections, we will delve deeper into the theoretical framework and practical applications of this topic.
Tips for Understanding Bond Order Molecular Orbitals
The following tips provide practical guidance for comprehending bond order molecular orbitals, enhancing your understanding of chemical bonding and molecular properties.
Tip 1: Visualize Atomic Orbital Overlap: Imagine the spatial arrangement of atomic orbitals and how they overlap to form molecular orbitals. This visualization helps grasp the relationship between orbital overlap and bond strength.
Tip 2: Analyze Electron Density Distribution: Examine the electron density distribution around atoms and bonds. High electron density indicates strong bonding interactions, while low electron density suggests weaker bonds or non-bonding interactions.
Tip 3: Relate Bond Order to Bond Strength: Understand the direct correlation between bond order and bond strength. Higher bond orders indicate stronger bonds, while lower bond orders correspond to weaker bonds, providing insights into molecular stability.
Tip 4: Consider Molecular Geometry: Recognize the influence of molecular geometry on bond order molecular orbitals. The arrangement of atoms in space affects orbital overlap and hence bond order.
Tip 5: Apply Quantum Mechanics Principles: Utilize the principles of quantum mechanics, such as wave-particle duality and the uncertainty principle, to understand the behavior of electrons in molecular orbitals.
Tip 6: Leverage Molecular Orbital Theory: Employ molecular orbital theory to construct molecular orbitals as linear combinations of atomic orbitals. This approach provides a framework for calculating and predicting bond orders.
Tip 7: Examine Hybridization Effects: Analyze how hybridization affects the shapes and energies of atomic orbitals, influencing their overlap and the resulting bond order molecular orbitals.
Tip 8: Consider Resonance and Delocalization: Understand the concepts of resonance and delocalization, where electrons are spread over multiple atoms or molecular orbitals, affecting bond order and molecular properties.
By incorporating these tips into your approach, you will develop a comprehensive understanding of bond order molecular orbitals, enabling you to predict and interpret various chemical phenomena.
In the following section, we will explore advanced applications of bond order molecular orbitals, demonstrating their significance in complex chemical systems and practical scenarios.
Conclusion
This article has provided a comprehensive exploration of how to find bond order molecular orbitals, elucidating the theoretical framework and practical applications of this concept. Key insights include:
- Bond order molecular orbitals offer valuable insights into bond strength, molecular properties, and chemical reactivity.
- Various factors such as atomic orbital overlap, electron density distribution, and molecular geometry influence bond order and molecular orbitals.
- Quantum mechanics, molecular orbital theory, and concepts like hybridization and resonance provide a deeper understanding of bond order molecular orbitals, enabling accurate predictions of molecular behavior.
Understanding bond order molecular orbitals is crucial for comprehending the electronic structure, stability, and reactivity of molecules. This knowledge finds practical applications in diverse fields, including materials science, catalysis, drug design, and biochemistry. As we continue to explore the intricate world of molecular bonding, the concept of bond order molecular orbitals will undoubtedly remain a cornerstone for unraveling the complexities of chemical systems and designing novel materials with tailored properties.